| Home | Energy Physics | Nuclear Power | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|
ORIGIN OF THERMAL RADIATION:
Matter is composed of molecules. The molecules have kinetic energy and in a solid or liquid may vibrate within mutual potential energy wells. In a gas molecules interact in collisions via their extended fields. Interacting atoms readily convert molecular kinetic energy into electron spheromak excitation energy or vice versa. The excited electrons occupy discrete energy states. An electron transiting from a discrete energy state to another discrete energy state does so by emission or absorption of a photon of a particular frequency.
Hence matter consists of an assembly of atoms within a sea of photons. At the surface of solid or liquid matter photons are constantly being emitted into the surrounding space and absorbed from the surrounding space.
At thermal equilibrium in an assembly of many interacting molecules thermal molecular kinetic energy is in equilibrium with electron spheromak excitation energy. This electron spheromak excitation energy is also in equilibrium with the photon frequency spectrum and flux. At steady state at constant temperature the average population of each electron energy state remains constant and there is a characteristic photon flux versus photon frequency distribution. This photon flux versus photon frequency distribution increases with temperature. This distribution normalized to radiated power versus frequency is known as the Planck distribution.
EQUAL TEMPERATURES:
If a solid is suspended by a thermally insulating fiber in an evacuated container with uniform temperature walls over time the suspended solid will acquire the same temperature as the container walls. There is a net exchange of radiant energy between the suspended solid and the container walls that falls to zero when the radiant energy flux emitted by the suspended solid equals the radiant energy flux absorbed by the suspended solid. At zero net radiation flux the temperature of the suspended solid equals the temperature of the surrounding container. For each such temperature there is a corresponding radiant energy flux per unit area that at steady state is both absorbed and emitted. Note that the surface reflectivity Fr affects the rate of energy absorption and the emissivity Ft affects the rate of energy emission. Hence if the emission spectrum is the same as the absorption spectrum then:
(1 - Fr) = Ft
If the container or an object is made such that it reflects no electromagnetic radiation (Fr = 0) and has no filtering atmosphere (Ft = 1.0) it is known as a "black body". For black bodies there is a simple relationship between temperature and thermal radiation density.
In general at energy balance:
(incident energy flux) (1 - Fr) = (ideal black body emission energy flux) (Ft)
FREQUENCY DEPENDENCE:
In some cases a molecule is formed from two or more elements that are held together by ionic bonding. In ionic bonding one atom gives up an electron to another atom so that the molecule has an electric dipole moment. If the molecule is in the gaseous state it can both vibrate and rotate. The frequency and amplitude of the molecular vibration or rotation is a function of the molecular structure and the material temperature.
As the molecule vibrates or rotates due to its electric dipole moment it can readily emit or absorb electromagnetic photons at its rotation or vibration frequencies. Within an assembly of many molecules different molecules types have characteristic frequency bands at which photons are readily absorbed and/or emitted. The rate of photon emission/absorption and the average photon frequency increase with temperature.
DEEP SPACE:
In deep space there are no walls. Using radio telescopes astronomers have determined that in deep space the average radiant energy density corresponds to black body walls with a temperature of about 2.7 degrees K. Most real objects such as planet Earth are much warmer than this and hence their equivalent black body surface temperature can be determined by analyzing the spectrum of the thermal radiant energy that they emit.
However, an atmosphere around a planet introduces complications. From an emitted thermal radiation perspective the surface of the planet is not at the solid-gas interface. From an emitted radiation perspective the surface of the planet is at the interface between the vacuum of space and the gas molecules that readily emit or absorb thermal frequency radiation. For CO2 and ozone gas molecules this interface is in the upper atmosphere and for H2O molecules this interface is at lower altitudes where clouds occur. On a crystal clear cloudless day the interface moves down to close to ground level.
Increasing the CO2 concentration in tha atmosphere effectively further raises the altitude at which CO2 molecules emit photons into deep space. At a higher altitudes the temperature is lower and hence the radiant energy emission is less. This reduction in radiant energy emission within the CO2 absorption band causes an increase in lower atmosphere temperature.
The thermal radiation emission from H2O in the atmosphere primarily occurs near 0 degrees C. However, a small increase in temperature near 0 degrees C causes a large decrease in H2O crystal solar reflectivity Fr (albedo). This decrease in albedo causes a further increase in lower atmosphere temperature.
MATHEMATICAL CHARACTERIZATION OF THERMAL RADIATION:
A photon is a packet of radiant energy. The energy E of a photon is given by:
E = h F
where:
h = Planck constant
and
F = radiation frequency.
Hence there is a direct proportionality between a photon frequency and photon energy.
All objects emit thermal electromagnetic radiation. The characterization of this radiation was one of the early successes of quantum mechanics.
Define:
h = 6.6256 X 10^-34 joule-sec = Planck's constant
K = 1.38054 X 10^-23 joule-K^-1 = Boltzmann constant
C = 2.997925 X 10^8 m / s = speed of light
Lamda = wavelength = C / F
Pi = 3.14159
W = 2 Pi F = photon angular frequency
From thermal theory, the radiated power Pr per unit surface area between angular frequencies W and W + dW emitted by a body with surface temperature T is given by:
Pr(W) dW = Ftw(W) [h / (8 Pi^3 C^2)] {W^3 dW /[exp((h W)/ (2 Pi K T)) - 1]}
where Ftw(W) is the frequency dependent emissivity
where:
0 < Ftw(W) < 1.0
Note that Ftw(W) = 1 for an ideal black body.
Reference: Fundamentals of Statistical and Thermal Physics by F. Reif, 1965, McGraw-Hill.
For an atmosphere around a planet Ftw(W) is of the form:
Ftw(W) = Exp[-(Coa(W) Pa + Cob(W) Pb + Coc(W) Pc + Cod(W) Pd)]
where:
Coa(W) = frequency dependent emission attenuation by GHG a;
Pa = partial pressure of GHG a;
Cob(W) = frequency dependent emission attenuation by GHG b;
Pb = partial pressure of GHG b;
Coc(W) = frequency dependent emisssion attenuation by GHG c;
Pc = partial pressure of GHG c;
Cod(W) = frequency dependent emission attenuation by GHG d;
Pd = partial pressure of GHG d.
Make substitution:
Z = [(h W) / (2 Pi K T)]
= [(h F) / (K T)]
Then:
dZ = h dW /(2 Pi K T)
Rearranging these equations gives:
W = (2 Pi K T Z) / h
or
W^3 = [(2 Pi K T Z) / h]^3
and
dW = (2 Pi K T dZ) / h
Hence:
Pr(W) dW
= Ftw(W) [h / (8 Pi^3 C^2)] {[(2 Pi K T Z) / h]^3 [(2 Pi K T dZ) / h] /[exp(Z) - 1]}
= Ftw(W) [(2 Pi (K T)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]
SPECIAL CASE OF AN IDEAL BLACK BODY WHERE Ftw(W) = 1:
For the special case of:
Ftw(W) = 1,
T = Te = black body surface temperature
then:
Pr(W) dW = Pro(W) dW
= [(2 Pi (K Te)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]
The variable component of this function is:
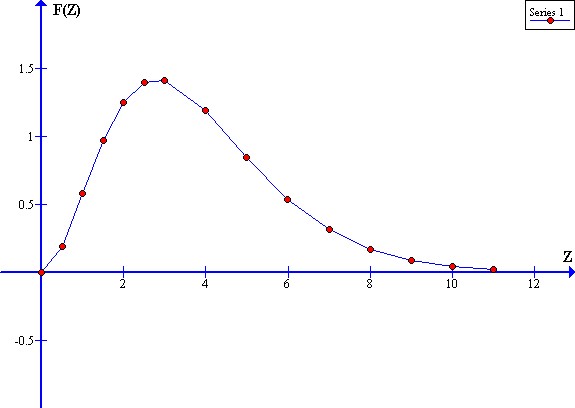
F(Z) = Z^3 / [exp(Z) - 1]
A graph of F(Z) versus Z gives:

This function has a power per unit frequency peak at:
Z = Zp = (h F) / (K Te) = 2.8214
where:
F(Zp) = [((2.8214)^3) / (exp(2.8214) - 1)]
= 22.45918466 / 15.80035471
= 1.4214
For Ftw(W) = 1:
About 3 / 4 of the infrared radiation power is within the range:
(Zp / 2) < Z < (2 Zp)
or
1.41 < Z < 5.64
For this special case of:
Ftw(W) = 1
the total radiated power P per unit area is given by:
P = [(2 Pi (KTe)^4)/(h^3 C^2)] Integral from Z = 0 to Z = infinity of:
{Z^3 dZ / [exp(Z) - 1]}
The definite integral from Z = 0 to Z = infinity of:
{Z^3 dZ / [exp(Z) - 1]}
= Pi^4 / 15
Hence:
P = [(2 Pi (K Te)^4)/(h^3 C^2)][Pi^4 / 15]
= (2 Pi^5) (K Te)^4/ (15 h^3 C^2)
= [(2 Pi^5 K^4) / (15 h^3 C^2)] Te^4
= Cb Te^4
where Cb is given by:
Cb = (2 Pi^5 K^4) / (15 h^3 C^2)
= 5.6697 X 10^-8 W m^-2 K^-4
= the Stefan-Boltzmann constant.
EXAMPLE:
Recall that for Ftw = constant the frequency of peak thermal radiative emission is given by:
(h F) / (K Te) = 2.8214
or
(1 / F) = h / (2.8214 K Te)
The wavelength of the peak in emitted power per unit frequency for a body with Ftw = constant and a surface temperature of 270 K is given by:
Lamda = C / F
= (C h) / (2.8214 K Te)
= (2.99792 X 10^8 m / s X 6.6256 X 10^-34 joule-s) / (2.8214 X 1.38054 X 10^-23 joule-K^-1 X 270 K)
= .018887 X 10^-3 m
= 18.887 um
About 3 / 4 of this infrared radiation power is within the wavelength range:
Lamda = 37.77 um to Lamda = 9.443 um.
REAL BODY:
Define Ft for a real body at actual temperature Ta by:
P = Cb Te^4 = Ft Cb Ta^4
or
Ft = (Te / Ta)^4
Thus Ta is the actual temperature that causes the same thermal power emission per unit area P as a black body at temperature Te.
For a real Earth with an atmosphere:
Ft < 1.
Hence:
Ta > Te
which causes an increase in emission temperature Ta due to the presence of an atmosphere with IR absorption bands.
For this case:
Ftw(W) is not constant
T = Ta,
Pr(W) dW
= Ftw(W) [(2 Pi (K Ta)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]
In this general case the radiated power per unit area is:
P = Integral from W = 0 to W = infinity of:
{Pr(W) dW}
= Integral from Z = 0 to Z = infinity of:
{Ftw(W) [(2 Pi (K Ta)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]}
= Ft Cb Ta^4
Solve for Ft:
Ft = Integral from Z = 0 to Z = infinity of:
{Ftw(W) [(2 Pi (K Ta)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]} / Cb Ta^4
= Integral from Z = 0 to Z = infinity of:
Ftw(W) {[(2 Pi K^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]} / Cb
= Integral from Z = 0 to Z = infinity of:
Ftw(W) {[(2 Pi K^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]} / {(2 Pi^5 K^4) / (15 h^3 C^2)}
= Integral from Z = 0 to Z = infinity of:
Ftw(W) {Z^3 dZ / [exp(Z) - 1]} / {(Pi^4) / (15)}
= Integral from Z = 0 to Z = infinity of:
{Pr(W) dW / [[(2 Pi (K Ta)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]]} {Z^3 dZ / [exp(Z) - 1]} / {(Pi^4) / (15)}
Recall that for a black body at temperature Ta:
Pro(W) dW = [(2 Pi (K Ta)^4)/(h^3 C^2)] Z^3 dZ / [exp(Z) - 1]
Hence:
Ft = Integral from Z = 0 to Z = infinity of:
{Pr(W) / Pro(W)}{Z^3 dZ / [exp(Z) - 1]} / {(Pi^4) / (15)}
= Integral from Z = 0 to Z = infinity of:
{15 / Pi^4}{Pr(W) / Pro(W)}{Z^3 dZ / [exp(Z) - 1]}
Note that for the theoretical special case of:
{Pr(W) / Pro(W)} = (constant)
Then:
Ft = Integral from Z = 0 to Z = infinity of:
{Pr(W) / Pro(W)} {15 / Pi^4}{Z^3 dZ / [exp(Z) - 1]}
= {Pr(W) / Pro(W)} {15 / Pi^4} {Pi^4 / 15}
= {Pr(W) / Pro(W)} = (constant)
as expected.
In general:
Ft = Integral from Z = 0 to Z = infinity of:
{Pr(W) / Pro(W)} {15 / Pi^4}{Z^3 dZ / [exp(Z) - 1]}
and
dFt = Integral from Z = 0 to Z = infinity of:
{15 / Pi^4} d{Pr(W) / Pro(W)}{Z^3 dZ / [exp(Z) - 1]}
where:
Z = (h F) / (K Ta)
These equations can be numerically evaluated to find the ratio (dFt / Ft). In the ratio the constant:
{15 / Pi^4} cancel.
Recall that:
P = Ft Cb Ta^4
dP = 0 = Ft Cb 4 Ta^3 dTa + dFt Cb Ta^4
Hence:
dFt / Ft = - 4 (dTa / Ta)
or
dTa = (Ta / 4) (- dFt / Ft)
This equation is used to calculate the change in atmospheric emission temperature caused by a change dFt in Ft.
Now consider two cases a and b corresponding to Ft = Fta and Ft = Ftb.
Recall that:
P = Fta Cb Taa^4 = Ftb Cb Tab^4
or
(Tab / Taa) = (Fta / Ftb)^0.25
or
(Tab - Taa) = Taa [(Fta / Ftb)^0.25 - 1]
This equation is more accurate for calculating large changes in temperature Ta.
It can be shown that:
Ftw(W) = Pr(W) / Pro(W)
= 1 / [1 + (Coa(W) Pa) + (Cob(W) Pb) + (Coc(W) Pc) + (Cod(W) Pd)]
where:
Pi = partial pressure of gas i;
Coi = frequency dependent infrared photon scattering cross section for gas i;
a = H2O
b = CO2
c = O3
d = CH4
The experimental data collected by a far infrared spectrometer permits evaluation of the quantities:
[Coa(W) Pa], [Cob(W) Pb], [Coc(W) Pc], [Cod(W) Pd].
Once these values are determined the effects of changes in individual GHG concentrations are easily determined. for example if the CO2 concentration is doubled then [Cob(W) Pb] is replaced by [2 Cob(W) Pb].
These equations relate Ft to Ftw(W), which is the frequency or wavenumber dependent emissivity.
Note that "wavenumber" is defined by:
Wavenumber = F / C
THERMAL POWER EMITTED BY AREA As:
For a real body with emitting surface area As at temperature Ta the thermal radiation emitted is:
Ft Cb Ta^4 dAs
ENERGY CONSERVATION:
Let Ac = Earth's cross sectional area absorbing solar radiation
Let As = Earth's surface area that emits infrared radiation energy captured by area Ac
Let Ho = solar irradiance at the Earth's orbit
Let Fr = Earth's planetary Bond albedo
Let Ha = net absorbed heat per unit surface area per unit time.
Assume that the heat conducted from the hot central core of the earth to its surface is negligibly small compared to the solar irradiance. Then energy conservation requires that:
(absorbed solar radiation) = (transmitted infrared radiation) + (net absorbed heat)
or
Ho (1 - Fr) Ac = Ft Cb Ta^4 As + Ha As
which is referred to herein as the Energy Conservation Equation. Note that Ft is measured at far infrared wavelengths and Fr is measured at solar spectrum (visible) wavelengths.
BLACK BODY MODEL:
The Energy Conservation Equation can be rewritten as:
Ho (1 - Fr) Ac = Cb Ta^4 As - (1 - Ft) Cb Ta^4 As + Ha As
Thus the Earth can be considered to be a black body surrounded by a partially reflecting filter. The incoming solar power is:
Ho Ac
of which:
Fr Ho Ac
is reflected back into space and
(1 - Fr) Ho Ac
is absorbed. The black body emits thermal radiative power is:
Cb Ta^4 As
of which
Ft Cb Ta^4 As
is transmitted into space and
(1 - Ft) Cb Ta^4 As
is reflected back and reabsorbed by the black body. Thus the total power absorbed by the black body is:
Ho (1 - Fr) Ac + (1 - Ft) Cb Ta^4 As
Energy conservation requires that this total power equals the absorbed thermal power
Ha As
plus the emitted black body radiation
Cb Ta^4 As
or
Ho (1 - Fr) Ac + (1 - Ft) Cb Ta^4 As = Ha As + Cb Ta^4 As
or
Ho (1 - Fr) Ac = Ft Cb Ta^4 As + Ha As
as before.
The importance of this black body representation is that Ta is the actual temperature of the equivalent black surface where the actual thermal radiative emission occurs. In the case of the Earth most of the thermal radiative emission is from heat released by condensation of water vapor and freezing of water micro-droplets at cloud level, so the average emission temperature Ta is the average temperature at cloud level, which is considerably less than the average ground level temperature Tag. On average:
(Tag - Ta) is about 18 degrees C.
HEAT ABSORPTION THEORM:
The following heat absorption theorm can be used to quantify the net rate of heat absorption caused by global warming in situations where the surface temperature is held constant by a phase change such as the melting of ice and/or by mixing within a large thermal mass.
The Energy Conservation Equation gives:
Ho (1 - Fr) Ac = Ft Cb Ta^4 As + Ha As
Differentiation of the Energy Conservation Equation gives:
Ho (-dFr) Ac = dFt Cb Ta^4 As + 4 Ft Cb Ta^3 dTa As + dHa As
for small variations in Fr, Ft, Ta and Ha.
If Ta = constant, then dTa = 0, giving:
Ho (-dFr) Ac = dFt Cb Ta^4 As + dHa As
or
dHa = [Ho (-dFr) (Ac / As)] - dFt Cb Ta^4
Consider the surface of a desert where:
Ha = 0
giving:
dHa = 0
giving:
Ho (-dFr) Ac = dFt Cb Ta^4 As + 4 Ft Cb Ta^3 dTa As
or
Ho (-dFr) (Ac / As) = dFt Cb Ta^4 + (4 dTa / Ta) (Ft Cb Ta^4)
Note that if both situations are at the same latitude the quantity:
Ho (-dFr) (Ac / As) - dFt Cb Ta^4
is the same.
Combining these two equations gives:
dHa (for Ta = constant) = (4 dTa / Ta) (Ft Cb Ta^4) (for Ha = 0)
Substitution of the Energy Conservation Equation into this equation gives:
dHa (for Ta = constant) = (4 dTa / Ta) [(Ho (1 - Fr) (Ac / As)] (for Ha = 0)
For a spherical Earth:
Ac = Pi R^2 = disc area
As = 4 Pi R^2 = surface area
Hence:
Ac / As = (1 / 4)
giving:
dHa (for Ta = constant) = (dTa / Ta) Ho (1 - Fr) (for Ha = 0)
This equation relates the global warming induced change in heat absorption rate at constant temperature (the ocean) to the global warming induced change in temperature with no net heat absorption (very dry land). The practical significance of this equation is that it permits calculation of dHa for oceans or for irrigated farm crop land from the change in average temperature dTa obtained from mathematical analysis of the Earth's thermal emission spectrum.
DRY LAND TEMPERATURE:
At very dry inland locations almost all of the absorbed solar radiation is retransmitted as infrared radiation, so that:
Ha ~ 0.
Hence, rearranging the energy conservation equation gives:
Ta = (Ho Ac / As Cb)^.25[(1 - Fr) / Ft]^.25
TEMPERATURE OF AN IDEAL BODY:
It is helpful to first investigate the theoretical temperature Te of an ideal spherical body with a partially reflecting surface. Assume that the reflectance Frw(W) and emissivity Ftw(W) of the surface are frequency independent.
Let Te = surface temperature of this ideal body.
With frequency independent reflectance and emissivity:
Frw(W) = Fr= constant
and
Ftw(W) = Ft = constant
Further assume that for this ideal body:
Ft = (1 - Fr)
so Fr and Ft satisfy:
Ft + Fr = 1
Then energy balance gives:
Ho Ac = Cb Te^4 As
or
Te = (Ho Ac / Cb As)^.25
This is the same temperature as for an ideal black body where Ft = 1 and Fr = 0.
ACTUAL EMISSION TEMPERATURE FOR Ha = 0:
For dry inland locations combining the above expressions for Ta and Te gives:
Ta = Te [(1 - Fr) / Ft]^.25
This expression shows the change in emission temperature from Te to Ta caused by non-ideal values of Fr and Ft. The emission temperature Ta is the cloud level temperature of a location on the Earth's surface where Ha = 0, as seen from outer space. Note that for the Earth much of the emitted thermal radiation originates at cloud level so the emission temperature Ta is generally less than the ground level temperature Tag. Most of the solar power absorption by the ocean causes evaporation which is energy balanced by thermal radiative emission from the condensing water vapor, so the net heat absorption or net heat emission is relatively small.
CHANGE IN THERMAL EMISSION TEMPERATURE WHEN THERE IS NO ONGOING NET HEAT ABSORPTION:
Recall that if Ha = 0 then:
Ta = Te [(1 - Fr) / Ft]^.25
or
Ta^4 = Te^4 [(1 - Fr) / Ft]
Differentiating this expression gives:
4 Ta^3 dTa = Te^4 d[(1 - Fr) / Ft]
= Te^4 [Ft (-dFr) - (1 - Fr) dFt] / Ft^2
= Ta^4 [Ft / (1 - Fr)][(-dFr / Ft) - ((1 - Fr) dFt / Ft^2)]
= Ta^4 [(-dFr / (1 - Fr)) + (-dFt / Ft)]
or
(4 dTa / Ta) = [(-dFr / (1 - Fr)) + (-dFt / Ft)]
Experimetal data collected by others indicates that:
1.7 dTa = dTag
Hence:
(4 dTag / (1.7 Ta)) = [(-dFr / (1 - Fr)) + (-dFt / Ft)]
or
dTag = (1.7 Ta / 4)[(-dFr / (1 - Fr)) + (-dFt / Ft)]
This equation quantifies the change in dry inland atmospheric temperature dTag caused by changes in Fr and Ft. Note that a decrease in Ft due to increased atmospheric CO2 concentration or increased atmospheric water vapor concentration will cause an increase in dry land temperature. Note that a decrease in Fr due to melting of cloud ice crystals will cause a further increase in dry land temperature.
Since Fr and Ft are functions of cloud cover, which varies both in time and location, the emission temperature Ta is locally highly variable. Temperature Ta will only seem to take on an average value when the whole disc of the earth is viewed so that local variations in Fr and Ft average out.
SPECIAL CASE of dFr = 0:
Experimental data seems to indicate that for Ta values far from Ta = 273.16 K the average planetary albedo Fr is nearly constant. Recall that:
(4 dTa / Ta) = [(-dFr / (1 - Fr)) + (-dFt / Ft)]
For the special case of dFr = 0 this equation simplifies to:
(4 dTa / Ta) = (-dFt / Ft)
or
(dTa / Ta) = (-dFt /4 Ft)
This equation relates the change in emission temperature dTa to the change in emissivity dFt when the change in planetary albedo dFr = 0.
EXACT EXPRESSION:
Assume that there is no net heat absorption or emission (Ha = 0). Then:
Ta = Te [(1 - Fr) / Ft]^.25
Assume that at some past time temperature Ta = Taa.
Assume that the present time temperature Ta = Tab.
Then:
(Tab / Taa) = [(1 - Frb) Fta / (1 - Fra) Ftb]^0.25
or
(Tab - Taa) = Taa [[(1 - Frb) Fta / (1 - Fra) Ftb]^0.25 - 1]
Note that this expression for (Tab - Taa) in terms of Taa, Fra, Frb, Fta and Ftb is exact for Ha = 0. Except for wind effects there are no assumptions or approximations. To the extent that a change in atmospheric composition affects measured values of Fr and Ft the effect of the change in atmospheric composition on Ta can be precisely calculated. However, the relationship between emission temperature Ta in the atmosphere and temperature Tag at ground level remains to be determined.
Experimental data collected by others indicates that:
(Tabg - Taag) = 1.7 (Tab - Taa)
where:
Tabg = dry ground temperature corresponding to Tab;
Taag = dry ground temperature corresponding to Taa.
Then:
(Tabg - Taag) = 1.7 Taa [[(1 - Frb) Fta / (1 - Fra) Ftb]^0.25 - 1]
This equation could potentially be used to evaluate future global warming if sufficiently accurate measurements of the changing values of Fr and Ft become available.
NET HEAT ABSORPTION BY THE POLAR OCEANS:
In the equitorial ocean lower density warm water floats on top of higher density cold water so the ocean surface temperature follows the average air temperature.
In the polar ocean, where the ocean surface temperature is less than 4 degrees C, warming of the ocean surface water by sunlight increases its density, which makes the warmer water sink. The ocean surface temperature is effectively held constant by this effect in conjunction with the proximity of floating ice. The deep ocean heat capacity is sufficiently large that the average ocean surface temperature changes only very slowly as compared to the rate of change in atmospheric greenhouse gas concentration. Then for ocean heat absorption calculation purposes the ocean surface temperature may locally be considered constant so the heat absorption rate per unit area dHa by the polar ocean is given by:
dHa (for Ta = constant) = (dTa / Ta) Ho (1 - Fr)
where dTa is the average temperature increase at a dry inland locations as determined by analysis of the Earth's infrared emission spectrum.
Let Ap = polar ocean area
Then the rate of heat absorption by the oceans is given by:
Ap dHa = (dTa / Ta) Ho (1 - Fr) Ap
This equation, which gives the net heat absorption rate by oceans, is of importance in calculating the polar ice melting rate and hence the future rate of increase in sea level.
EXTRA IRRIGATION REQUIREMENTS:
One of the functions of farm crop irrigation is to maintain crop temperature for optimum growth and to prevent crops being damaged or destroyed on hot days. Irrigation water provides cooling by evaporation. The resulting water vapor is wind borne far from the farm before the water vapor condenses. The latent heat released during condensation contributes to the planetary infrared emission, so evaporation of irrigation water does not directly contribute to the net planetary heat absorption rate. The increase in required crop irrigation per unit time due to global warming is given by:
dHa / (Ro Hv)
where:
dHa = increase in heat absorption per crop unit area per unit time due to global warming
Ro = density of water (1000 kg / m^3)
Hv = latent heat of vaporization of water (2270 kJ / Kg)
Recall that:
dHa = (dTa / Ta) Ho (1 - Fr)
where dTa is obtained from analysis of the Earth's infrared emission spectrum.
Hence, the increase in required crop irrigation due to global warming is given by:
dHa / (Ro Hv) = (dTa / Ta) Ho (1 - Fr) / (Ro Hv)
This increase in required crop irrigation has huge practical consequences.
This web page last updated April 20, 2017
| Home | Energy Physics | Nuclear Power | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|